ooh! OOH! ESCAPE!
There is no doubt that escaping capture was critical to survival.
So, what starts escape and also other forms of movement? Let’s start by looking at a primitive animal: the leech.
Escape for the leech is swimming, as long as it is in relatively deep water. A tap on its tail will result in fast swimming!
How does a leech swim? It creates an approximately sinusoidal, undulatory traveling wave. Contraction and relaxation of dorsal and ventral longitudinal muscles are primarily responsible for swimming undulations (Lamb & Calabrese, 2011).
NOTE: for scientists, there is neural activity ‘sign’ that an isolated or nearly isolated leech preparations would be swimming if it was in an intact leech. The ‘sign’ is a bursting pattern of a dorsal excitatory motoneuron in these preparations, and the behavior is called “fictive” swimming. Researchers frequently refer to “fictive swimming” as a “swim”, “swim episode”, “swimming”, and “swim activity” (Mullins et al. 2011a).
What are the pathways activated in these muscles? At the base is a central pattern generator (CPG) circuit that is composed of complex segmental oscillators which rely heavily on intersegmental connectivity (Lamb & Calabrese, 2011).
Midbody ganglia contain a bilateral, triphasic oscillator CPG circuit composed predominately of bilaterally paired interneurons. And the output from these oscillator interneurons controls the activity of the excitatory and inhibitory motoneurons. It is these interneurons that provide the final common pathway to the longitudinal muscles used for swimming. Otherwise, a leech is simply resting.
Okay, then what depolarizes the oscillators initially in the swim burst? Unpaired cells, number 204, located also in midbody ganglia, appear to be briefly depolarized, opening a gate on its axon along which spikes travel to evoke the oscillatory burst that drive oscillation until some other process terminates swimming. These command gating neurons are in turn activated by trigger neurons in the rostral (head) brain or by a ‘novel’ cell, E21, in the rearmost midbody ganglion next to the caudal (tail) brain. Cell E21 has only a rostrally (headward) projecting axon that connects to all the other ganglia; it has been labeled as “trigger command neuron” (click link for PDF: Mullins et al. 2011b).
The ability of leech cell E21 to elicit swimming is shown in Figure 1. The drawing shows the head region is intact and the location of the 21st ganglion, next to the tail (T) brain. Stimulation of cell E21 can initiate swimming (A) and prolong it (B).
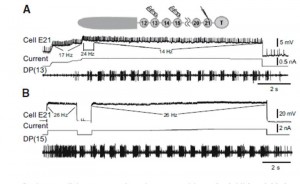
Figure 1. Physiologically appropriate cell E21 activity elicits swimming
in semi-intact preparations. Insert: experimental setup for both A
and B. A: impulse frequencies comparable to those elicited from body
stimulation initiated (24 Hz) and maintained (14 Hz) swimming.
B: moderate frequency cell E21 activity (26 Hz) initiated and extended
swimming. This swim was 80% longer than the longest control swim
in this preparation. (From Mullins et al. (2011b) Fig. 7.)
In reality, does cell E21 get any kind of sensory information? YES, in fact, cell E21 receives bilateral mechanical receptor inputs from all midbody ganglia (Mullins et al. 2011b). Cell E21 in turn integrates this information and can sent action potentials to the gating cells in ganglia 9 – 16 which then direct spikes to oscillator cells (Figure 2). They activate the motoneurons for swimming.
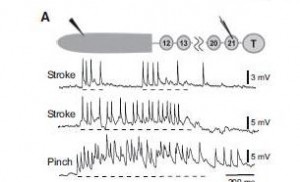
Figure 2. Body wall stimulation activates cell E21 A: activity in cell E21 during stroking and pinching of the rostral body wall in a semi-intact preparation Insert: experimental preparation; the shaded rostral body wall is nearly intact. Dashed line denotes the approximate timing of the stimulus. Note that the more noxious stimuli (pinching) elicited a higher impulse frequency. (From Mullins et al. (2011b) Fig. 5.)
Notice in Figure 3, neither the head brain nor the tail brain are involved in initiating swimming.
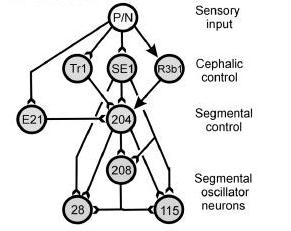
Figure 3. Brainstem structure that control swim initiation in lamprey. Note that E21 is not in the cephalic (head) control level; instead cell E21 in one segmental ganglion. Cell 204 is the command trigger cell that activates the cell 208, cell 28, and cell 115 segmental oscillator interneurons in the segmental ganglia.
In fact, the general model for locomotion is shown in Figure 4 and will be the discussed in future posts.
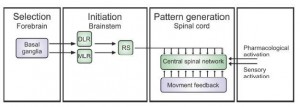
Figure 4. Schematic of the motor control structure common to all vertebrates. The selection of motor programs takes place in the basal ganglia in the forebrain. Locomotion is initiated by stimulation of diencephalic (DLR) and mesopontine (MLR) locomotor regions, which project independently to reticulospinal (RS) system neurons that provide excitation to the CPG. Sensory feedback generated by the ongoing movement can help to fine tune the different phases of locomotion. Experimentally locomotion can be induced by the application of pharmacological agents or activation of sensory input. (Modified from Eklof Ljunggren Thesis, 2012 Figure 1 (modified from Grillner, 2003))
For an expanded discussion about the initiation of locomotion, go to this review page on this website.
The take home is a ‘no brainer’; no, for excape behaviors and other similar locomotor behaviors, the higher centers of the brain is not utilized.
Next, are the motoneurons like those involved in swimming, for example, by zebrafish, recruited in a graded manner? Then, are the interneurons also recruited in a graded manner?
References
Eklöf Ljunggren E. (2012) “Organization of the spinal locomotor network in zebrafish: Pattern of recruitment and origin of excitatory drive.” (Doctoral dissertation). Retrieved from https://publications.ki.se/xmlui/bitstream/handle/10616/41277/Thesis_Emma_Ekl%C3%B6f_Ljunggren.pdf?sequence=2
Grillner S. (2003) The motor infrastructure: from ion channels to neural networks. Nat Rev Neurosci 4: 573-586.
Lamb DG, Calabrese RL. (2011) Neural circuits controlling behavior and autonomic functions in medicinal leeches. Neural Systems & Circuits 1: 13-21.
Mullins OJ, Hackett JT, Buchanan J, Friesen WO. (2011a) Neural control of swimming behavior: comparison of vertebrate and invertebrate model systems. Prog Neurobiol 93: 244-69.
Mullins OJ, Hackett JT, Friesen WO. (2011b) Local-distributed integration by a novel neuron ensures rapid initiation of animal locomotion. J. Neurophysiology 105: 130-144.