Student: What a pile of articles! And what did you find on commanding movement?
Professor: It depends upon:
- What are the types of movements?
- What is the nature of the network(s) for motor command?
Student: Well, one way of looking at movements is to consider the muscle mass involved:
- Fine motor movements, like tapping on a button, or
- Gross motor movements, like doing deep knee bends.
Professor: Then another view of movement is how much learning is involved:
- None, or
- Over-learned involving:
- Maintenance actions, like running smoothly at an easy pace,
- Reflexive actions, like walking over tilted stones on a pathway, or
- Voluntary actions, like switching to a different trail when running in the woods.
Professor: Now, what kind of motor command is involved with each?
Student: Interesting! Is this a trick question?
I thought that the only motor command is from Betz cells sending discharges down the corticospinal pathway.
Professor: Motor imagery (MI) studies have examined the role of the corticospinal pathway during imagined and actual movements. In a very comprehensive review, Guillot et al. (2012) found that:
“The contribution of the contralateral primary motor cortex (cM1) to imagined actions is […] controversial.”
- They reported that some researchers did not report any cM1 activations during MI, others found fleeting involvement, or significant activation and
- that, “Such discrepancies may be due to methodological differences and difficulties in monitoring compliance with MI instructions (Sharma et al., 2006).”
- “Taken together, the bulk of neuroimaging studies suggest that cM1 is activated during MI – but more weakly than during actual movement.
- Interestingly, Kasess et al. (2008) reported that SMA may substantially contribute to inhibit activity of cM1 during MI.”
Professor: These studies used mostly tasks involving fine motor actions like finger tapping and grosser movements like elbow flexion/extension.
Concerning voluntary, whole body movements, Zaaimi et al. (2012) made an extensive, unilateral sectioning of the medullary corticospinal fibers in the pyramidal tract in three adult monkeys. They found that:
- After an initial contralateral flaccid paralysis, all motor functioning recovered rapidly.
- After 3 – 11 days, all monkeys “were capable of leaping accurately across the holding pen from one perch to another, and could rapidly walk and climb.” Each could support their weight by gripping the cage bars with either or both hands “almost normally“.
- However, weak grip was observed using” contralesional hand.”
- Otherwise, “recovery of function was in accord with the extensive previous literature [beginning with] Lawrence and Kuypers (1968)“.
Student: Well, well; the corticospinal tract may not be a part of walking, running, or cycling!
Professor: Maybe not. If possibly not, then what other motor command networks may have been involved. In fact, Macuga and Frey (2012) wrote about “Hierarchical Organization of Neural Representations for Observation, Imagery and Imitation”:
“Our findings are generally consistent with a hierarchy in which areas showing increased activity during observation are a subset of those engaged during motor imagery, which in turn are a subset of those engaged during synchronous imitation.
- “Synchronous imitation resulted in greater increases than either imagery or observation in
- bilateral sensorimotor cortex, cerebellum, classic SMA, parietal operculum (putative secondary somatosensory cortex), and in several motor-related subcortical [(thalamus, putamen)] areas.
- These differences likely reflect processing of the descending efferent signal and afferent feedback.
- “Relative to synchronous imitation, observation is associated with greater increases in caudal SMA activity.
- “Relative to observation, motor imagery produced increased activation in pre-SMA, cingulate, anterior insula, and left inferior frontal cortex.”
Professor: Please remember that Kasess et al. (2008) reported that increased SMA activity may indicate an inhibition of cM1during imagery.
Student: How can we put these structures together into parts of a motor command network?
Professor: Here is a table of the components for the Motor Command Network.
| Motor Command Network Components | Pathway | Function | Source |
| Corticospinal Connections | Primary motor cortex to spinal cord interneurons | Fine motor control, gross motor control(?) | Zaaimi et al. (2012) |
| Corticoreticular Connections | Mainly prefrontal cortex to ponto- medullary reticular formation | Anticipatory postural adjustments during stepping | Yeo et al. (2012) |
| Fronto-striatal Connections | Primary motor cortex & DLPFC to putamen; VLPFC & SMA to caudate | Movement initiation & visually guided limb movement | Scherder et al. (2012) |
| Fronto-cerebellar Connections | Primary motor cortex, premotor, & SMA to medial cerebellum; IFG & DLPFC to lateral cerebellum | Timing & spatial coordination of lower limbs | Scherder et al. (2012) |
| Cingulum Connections | Cingulate gyrus to parahippocampal gyrus and uncus of the temporal lobe. | Stride-to-stride control of walking | Scherder et al. (2012) |
DLPFC, dorsolateral prefrontal cortex; VLPFC, ventrolateral prefrontal cortex; SMA, supplementary motor cortex; IFG, inferior frontal gyrus.
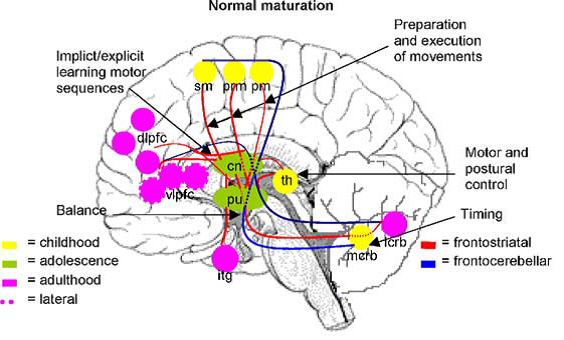
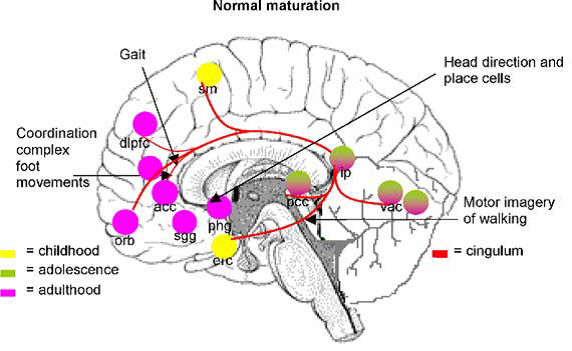
Professor: Several components are shown in Fig. 2A and Fig. 3A in the review of Scherder et al. (2012):
Fig. 2. Some major gait-related functions of brain regions belonging to the fronto-striatal and fronto-cerebellar connections during normal maturation. pm: primary motor cortex; prm: premotor cortex; sm: supplementary motor cortex; dlpfc: dorsolateral prefrontal cortex; vlpfc: ventrolateral prefrontal cortex; cn: caudate nucleus; pu: putamen; th: thalamus; mcrb: medial cerebellum; lcrb: lateral cerebellum, ifg; inferior frontal gyrus.
Fig. 3. Some major gait-related functions of brain regions belonging to the cingulum during normal maturation. sm: supplementary motor cortex; dlpfc: dorsolateral prefrontal cortex; acc: anterior cingulate cortex; orb: orbitofrontal cortex; sgg: subgenual anterior cingulate; phg: parahippocampal gyrus; erc: entorhinal cortex; pcc: posterior cingulate cortex (retrosplenial cortex); ip: inferior parietal lobe; vac: visual association cortex.
Student: I am going to redraw Fig. 11 for Proske U, Gandevia SC. (2012)!
Professor: And I will look up where exafference copy, proprioceptive feedback, and possible memory connect in their “difference calculator”. See you next week.
References
Guillot A, Di Rienzo F, Macintyre T, Moran A, Collet C. (2012) Imagining is not doing but involves specific motor commands: a review of experimental data related to motor inhibition. Front Hum Neurosci; 6:247. doi: 10.3389/fnhum.2012.00247.
Kasess CH, Windischberger C, Cunnington R, Lanzenberger, R, Pezawas L, Moser E.(2008).The suppressive influence of SMA on M1 in motor imagery revealed by fMRI and dynamic causal modeling. Neuroimage 40, 828–837.
Proske U, Gandevia SC. (2012) The proprioceptive senses: their roles in signaling body shape, body position and movement, and muscle force. Physiol Rev 92: 1651–1697.
Sharma N, Pomeroy VM, Baron JC.(2006).Motor imagery: a back-door to the motor system after stroke? Stroke 37, 1941–1952.
Yeo SS, Chang MC, Kwon YH, Jung YJ, Jang SH. (2012) Corticoreticular pathway in the human brain: diffusion tensor tractography study. Neurosci Lett Feb 2;508(1):9-12. doi: 10.1016/j.neulet.2011.11.030..
Zaaimi B, Edgley SA, Soteropoulos DS, Baker SN. (2012) Changes in descending motor pathway connectivity after corticospinal tract lesion in macaque monkey. Brain. February 1; 59(3): 2798–2807. doi:10.1016/j.neuroimage.2011.09.083.