SUMMARY: This post explores sensory gating of external and internal sensation. Can our reticular thalamic nuclei keep out or reduce ‘irrelevant’ sensory information for the endurance athlete?
EVIDENCE FOR SENSORY GATING
Student: That summary looks familiar: gating in sensory information and gating out other sensations.
Professor: Yes. (See more at: The Sensory Gate is Real.) First, let’s briefly review the evidence for sensory gating (SG). .
- The ability to do pre-attentive filtering of background sounds (Kisley et al, 2004).
- The loss of filtering ability due to strokes which include the ventral posterior lateral thalamic nuclei (VPL) and results in flooding of sensory gates (Staines et al., 2002).
- The second of paired inspiratory occlusions is reduced (Chan et al., 2008).
- The thalamus has a thin ‘skin,’ the reticular nuclei, whose function is to help focus our attention, allowing only ‘unusual’ activity to pass through gates (Halassa et al., 2014; Ward, 2013).
INDIVIDUAL DIFFERENCES IN SENSITIVITY
Student: Another way of looking at SG evidence is to notice individual differences in sensitivity.
Professor: The evidence does show that some individuals have
- too much sensorimotor sensitivity: anxious persons are very aware of background sounds, and/or
- too little sensorimotor sensitivity: depressed individuals show too little awareness (Paulus & Stein, 2010).
Student: Are there any scales for measuring individual sensorimotor sensitivity?
Professor: Yes. Hedrick and co-workers (2012) created a Sensory Gating Inventory and developed it to measure symptoms that refer to perceptual anomalies
- in the modulation of exteroceptive inputs (the Perceptual Modulation factor),
- in focusing attention (the Distractibility factor),
- of over-inclusion or hyper-attention (the Inundation factor), and
- during periods of fatigue and stress (the Fatigue and Stress Vulnerability factor).
Professor: Note: Kinsley et al. (2004) used an early Sensory Gating Inventory version for their 2004 study cited above.
POSSIBLE MECHANISMS
Student: Their Sensory Gating Inventory methodology reminds me of how you and your colleagues developed the Physical Activity Questionnaire (Weiser et al., 1973). And, neurophysiologically, how can these attentional changes happen?
Professor: Well, to explain this, let’s start by cutting a tangerine in half, across its sections. The sections look like triangles, like the shapes of thalamic nuclei. And around the tangerine is a skin, like the envelope that the reticular nuclei make around the dorsal thalamus.
- The thalamic ‘skin’ acts like a ‘sieve’ or ‘filter’ to only allow selective thalamic output to travel on towards the limbic structures and then towards the cortex.
- As Crick (1984) has written, “If the thalamus is the gateway to the cortex, the reticular complex might be described as the guardian of the gateway.”
- Various researchers have described insula as having “Searchlights” or “Hotspots” or “Attentional Doorways.”
- Changes in reticular neuron ion channels open or close “Doorways” by regulating the coupled calcium and potassium channels.
- Damages to these coupled channels could lead to highly leaky gateways for schizophrenics or to tightly closed gateways for depressed persons.
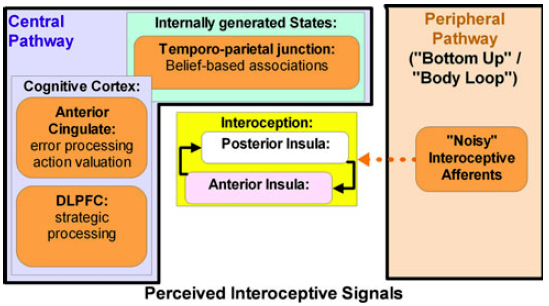
Student: The position of the thalamus is remarkable, and you have written that it has a highly effective interaction with the insula in the thalamocortical network. In fact, please look at the figure (below) that I just found.
Professor: Gee, that is Figure 1 from the Paulus & Stein (2010) model for anxiety. They call it a conceptual model, not a sequential model.
- Briefly, the black lines around the Peripheral Pathway[s] box could represent the reticular thalamic nuclei.
- The reticular thalamic nuclei doorways allow some of the ‘‘noisy’’ afferents to bring interoceptive information to the posterior insula.
- Higher cortical regions combine anterior cingulate and dlPFC processing with belief-based associations.
- They suggest these interactions are an attempt of the cognitive control apparatus to differentiate predictive from non-predictive signals.
Professor: And it’s similar to comments made by Farb et al. (2013), who examined internal versus external forms of attention. In a previous blog post, Symptoms spotted by Posterior Insula’s Searchlight, I quoted these investigators:
- “Respiratory [Interoceptive Attention] modulated
- a posterior insula region sensitive to respiratory frequency,
consistent with [a] primary interoceptive cortex, - [and] the ventral posterolateral and ventral posteromedial nuclei of the thalamus were highly connected with right posterior insula.
- a posterior insula region sensitive to respiratory frequency,
- The authors referred to this section of the thalamus as the “putative interoceptive thalamus”.
Professor: Furthermore, it seems like there is
- an “intero-proprio-ceptive network” composed of spinal and brainstem sensory tracts, thalamic relay nuclei, the thalamic reticular complex, and the posterior insula.
- This intero-proprio-ceptive network then links into both the more general cortico-thalamo-cortical network and salience network.
Student: For depressed persons, I suppose the opposite can be found, that the posterior insula is deactivated.
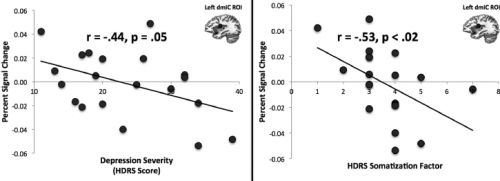
Professor: In fact, Avery et al., (2014) showed that in persons with a major depressive disorder,
- attention to heart rate was deactivated compared to ‘healthy controls’ and that
- depression severity was strongly correlated with deactivation of limbic system regions;
- shown in their figure above, the dorsal mid-insula cortex (dmIC) activation during heartbeat interoception, minus during visual exteroception, was negatively correlated with
- the depression severity determined on the Hamilton Depression Rating Scale (HDRS score) and
- the severity of somatic symptoms measured on a HDRS subscale.
Professor: Thus, individual sensitivities of their reticular thalamic nuclei and of the monitoring by their mid- and posterior-insula can be quite different.
INNERVENTIONS OF SENSORY GATING SENSITIVITY
Student: Are there interventions that can reduce symptoms, for example, muscle pain?
Professor: Meditators can manipulate ‘awareness’ of pain, compared to novices. For example, Lutz et al. (2013) found that expert meditators reported equal thermal pain intensity, but less unpleasantness.
- Again, this difference was associated with enhanced activity in
- the dorsal anterior insula and
- the anterior mid-cingulate
- which form the so-called ‘salience network,’ but only for meditation experts.
- A pattern of low baseline activity coupled with high response in anterior insula and anterior mid-cingulate was associated with enhanced neural habituation for experts only
- in anticipation of painful stimulation in the amygdala and other pain-related regions and
- during painful stimulation in the pain-related regions.
Professor: These findings suggest that cultivating experiential openness down-regulates anticipatory representation of aversive events, and increases the recruitment of attentional resources during pain, which is associated with faster neural habituation.
Student: Have any investigators studied up- and down-regulation of anterior insula and anterior mid-cingulate during real-time fMRI neurofeedback?
Professor: That’s a rhetorical question! Real-time neurofeedback is a ‘hot topic’ and has shown that
- down- and up-regulation for both anterior insula and anterior mid-cingulate can
- increase or decrease pain intensity and unpleasantness (Sulzer et al., 2012).
Student: Are there interventions for persons merely interested in being physically fit?
Professor: Yes. A body scan meditation is easy to do and can find muscle ‘knots.’ Then, deep imagery can help loosen them and enhance physical and mental performance.
Professor: For example, Mirams et al. (2013)
- used a period of brief body-scan mindfulness meditation training and
- found reduced tactile misperception with increased sensitivity during a somatic signal detection task.
Student: What is brief body-scan mindfulness meditation training?
Professor: Their meditation training was delivered using two fifteen minute audio recordings of a guided body-scan meditation exercise edited from a longer script (used previously by MacIver et al., 2008).
- Participants were started by focusing their attention on sensations of breathing in the chest, nostrils and throat; then
- in version one, participants directed their attention to their legs, feet and toes, pelvis, hip bones, and abdomen, lower and upper back and chest;
- in version two, participants were directed their attention to their neck and shoulders, arms, hands and fingers, head and face, eyes and mouth.
- Throughout each recording, participants were encouraged to notice any sensations in each body area and to observe what was happening with their thoughts and their bodies, moment by moment, without judgment or criticism.
Student: In fact, wasn’t phantom pain the focus of the study by MacIver et al. (2008)?
Professor: Indeed it was, and they tested the effect of brief body-scan mindfulness meditation training in patients with phantom pain. Following training, patients reported for their constant pain, a significant reduction in both
- intensity and
- unpleasantness
Take Home Points:
- The reticular thalamic nuclei ‘openness’ varies greatly between individuals and over time.
- The porous reticular thalamic nuclei border of certain individuals does have
- fewer sensory gates or doorways that are actually ion channels or
- lower percentage of open ion channels.
- Posterior insula
- acts as a monitor of open reticular thalamic nuclei gates/doorways and
- activates anterior insula and rest of the salience network.
- Pain and other symptoms can be reduced by meditation practices including body scan by up-regulating insula and mid-cingulate cortices.
Next:
The intensity of my perceptions is created in my mid-insula and can be reported by an elaborate introspective cortical process.
References:
Avery JA, Drevets WC, Moseman SE, Bodurka J, Barcalow JC, Simmons WC.(2014) Major depressive disorder is associated with abnormal interoceptive activity and functional connectivity in the insula. Biol Psychiatry 76: 258–266. DOI: 10.1016/j.biopsych.2013.11.027.
Chan P-YS. Davenport PW. (2008) Respiratory-related evoked potentials of respiratory sensory gating. J Appl Physiol 105: 1106-1113. DOI: 10.1152/japplphysiol.90722.2008.
Crick F (1984) Function of the thalamic reticular complex: The searchlight hypothesis. PNAS USA 81: 4586-4590.
Farb NAS, Segal ZV, Anderson AK.() Attentional modulation of primary interoceptive and exteroceptive cortices. Cereb. Cortex (2013) 23 (1): 114-126. DOI: 10.1093/cercor/bhr385.
Halassa MM, Zhe Chen Z, Wimmer RD, Brunetti PM, Zhao S, Zikopoulos B, Wang F, Brown EN, Matthew A. Wilson MA. (2014) State-dependent architecture of thalamic reticular subnetworks. Cell 158: 808–821. DOI: 10.1016/j.cell.2014.06.025
Hetrick WP, Erickson MA, Smith DA.(2012) Phenomenological Dimensions of Sensory Gating. Schizophrenia Bulletin 38: 178–191. DOI: 10.1093/schbul/sbq054.
Kisley MA, Noecker TL, Guinther PM. (2004) Comparison of sensory gating to mismatch negativity and self-reported perceptual phenomena in healthy adults. Psychophysiology 41: 604–612.
Lutz A, McFarlin DR, Perlman DM, Salomons TV, Davidson RJ. (2013) Altered anterior insula activation during anticipation and experience of painful stimuli in expert meditators. NeuroImage 64: 538–546. DOI: 10.1016/j.neuroimage.2012.09.030.
MacIver K, Lloyd DM, Kelly S, Roberts N, Nurmikko T. (2008) Phantom limb pain, cortical reorganization and the therapeutic effect of mental imagery. Brain 131: 2181-2191. DOI:10.1093/brain/awn124.
Mirams L, Poliakoff E, Brown RJ, Lloyd DM. (2013) Brief body-scan meditation practice improves somatosensory perceptual decision making. Consciousness and Cognition 22: 348–359. DOI: 10.1016/j.concog.2012.07.009.
Paulus MP, Stein MB (2010) Interoception in anxiety and depression. Brain Struct Funct 214: 451–463 DOI 10.1007/s00429-010-0258-9.
Staines WR, Black SE, Graham SJ, McIlroy WE. (2002) Somatosensory gating and recovery from stroke involving the thalamus. Stroke 33: 2642-2651. DOI:10.1161/01.STR.0000032552.40405.40.
Sulzer J, Haller S, Scharnowski F, Weiskopf W, Birbaumer N, Blefari ML, A.B. Bruehl AB, Cohen LG, deCharms RC, Gassert R, Goebel R, Herwig U, LaConte S, Linden D, Luft A, Seifritz E, Sitaram R. (2013) Real-time fMRI neurofeedback: Progress and challenges. NeuroImage 76: 386–399. DOI:.10.1016/j.neuroimage.2013.03.033.
Ward LM. (2013) The thalamus: gateway to the mind. WIREs Cogn Sci 4: 609–622. DOI: 10.1002/wcs.1256.
Weiser PC, Kinsman RA, Stamper DA. (1973) Task-specific symptomatology changes resulting from prolonged submaximal bicycle riding. Medicine and Science in Sports 5: 79-85.