Student: How young can a child be…. Well, how well coordinated can a child be so it can adapt to walking on a split-belt treadmill? Ahh, with a minimum of assistance?
Professor: Musselman et al. (2011) determined that children, older than 8.5 months, were the successful participant in thein study. Below is their Fig. 1 showing and describing their methods:
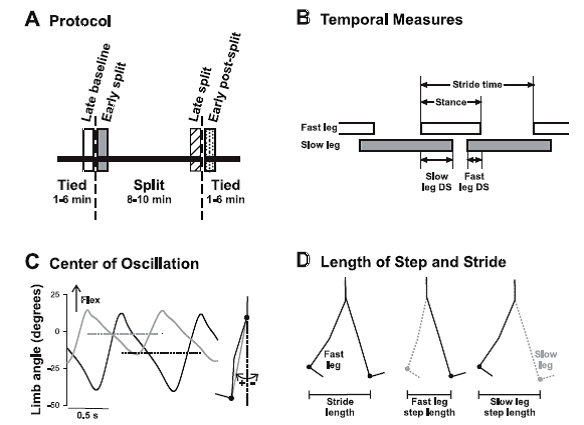
Fig. 1. Methods. A: experimental protocol. Children walked on a split-belt treadmill with the belts at the same speed (tied), followed by the belts at different speeds (split), and finally again in the tied condition. Time periods of interest are late baseline (open bar), early split (shaded bar), late split (hatched bar), and early postsplit (stippled bar) at 40 steps for each period. B: temporal measures of walking are shown: stride time, stance time, and double support time. Open and shaded horizontal bars indicate the duration of the stance phase, and the space between the bars represents the duration of the swing phase. The duration of a stride includes a stance and a swing phase. Temporal coordination was quantified by double support times (i.e., time when both feet are in contact with the ground), shown for when the slow leg is trailing (slow DS) and when the fast leg is trailing (fast DS). C: center of oscillation is the mean limb angle over a stride. Limb angles of the fast (solid line) and slow (shaded line) legs are plotted for 1 child (35.2 mo) during early split. Dashed horizontal solid and shaded lines represent the mean limb angles for the fast and slow legs, respectively. Limb angle is the angle between the vertical and a vector connecting the hip and ankle markers (shaded line in inset at right). D: step length and stride length are illustrated. Step length, defined as the distance between the ankle markers of the 2 legs in the anteroposterior direction, was measured at the time of foot contact of the leading limb (i.e., instant in time illustrated at middle and right). The step lengths are named according to the leading leg, by convention. Stride length (left) is the distance traveled in the anteroposterior direction by the ankle marker of a single leg through the stance phase (i.e., foot contact to lift off, limb position shown for the 2 instances in time). [Emphases added.]
Right-click this link to download PDF of their study
Professor: Pardon me; I want to clarify their dazzling biomechanical methodology. First, the sampling sequence for their data collection was:
- At the end of baseline when the belts were operating at the same speed (late baseline),
- Immediately after the belts were operating at different speeds (early split),
- At the end of split-belt walking (late split), and
- Then immediately after resuming the same speed (early post split).
Center of oscillation symmetry was their measure of spatial coordination – “where the limbs were placed during locomotion” (Malone and Bastian, 2010). Where the leg was oscillating around a vertical line through the hip, the stride motion was oscillating about a neutral, symetrical axis. Where the stride motion was oscillating about a flexed and ahead of the hip, the limb angle was positive and preparing for foot strike, and where limb angle was negative, one’s leg is extended, behind the hip, going through swing. The center of oscillation is defined as the midpoint of the limb angle between foot strike and toe off for each foot. The degree of center of oscillation symmetry was calculated from the mean value for the “fast” subtracted from the “slow” leg. If the symmetry value was positive, the leg had an asymmetrical oscillation about a flexed vertical axis, was negative, the leg had an asymmetrical oscillation about an extended axis, or was zero, “stepping in the spatial realm was symmetrical” about neutral vertical axis(Malone et al. 2011).
Step symmetry was their measure of temporal coordination – “when the limbs were placed” (Malone and Bastian, 2010). The value for step symmetry was calculated by subtracting the step lengths of the “fast” and “slow” leg. Then the difference was divided by the sum of the step lengths, so subjects of different height who take different sized steps could be compared. Therefore, it is a normalized difference between the step lengths of the “fast” and “slow” leg. A value of zero indicated steps are symmetrical; a positive symmetry value meant the fast step was larger than the slow step, and vice versa for negative values (Malone and Bastian, 2010). In the Musselman et al. (2011) study, symmetry values were also calculated for double support.
Student: Enough! Just what did the kids do when they were on the split-belt treadmill?
Professor: Okay. Below is Fig. 2 showing Time in Stance and Stride Length results from Musselman et al. (2011):
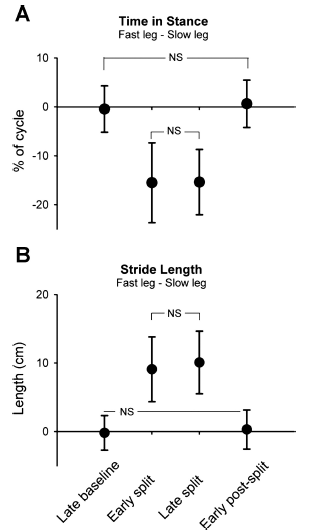
Fig. 2. Stance time and stride length. Group data (n = 27) are shown for time in stance (expressed as % of step cycle; A) and stride length (in cm; B). Data points represent mean values (from 40 steps) for each time period with error bars (SD). NS, not statistically significant. All other comparisons are significant (P < 0.008, Bonferroni).
Professor: Note that the values plotted in Fig. 2 are differences calculated as absolute values: % cycle for the stance time and length for stride length for the “fast” minus the time for the “slow” leg. An immediate increase in speed for the child’s leg on the fast belt was handled by a shorter stance time. This adaptation was maintained over the duration of split-belt walking. And amazing for these infants, as soon as the belts resumed equal speeds, the stance time immediately increased and was not different from the late baseline value.
Student: NO AFTEREFFECT! That indicates NO LEARNING, just adapting!!!!
Professor: The authors then state that,
“The duration of the stance phase and the excursion of the limb (i.e., stride length) are regulated by movement-related afferent feedback that affects phase transitions (i.e., stance to swing, swing to stance). For example, sensory inputs related to hip position and ankle loading regulate phase durations and transitions during locomotion (reviewed in Pearson 2008; Rossignol et al. 2006). Together, the results suggest that neural substrates controlling stance time and stride length in split-belt walking are contained in parts of the nervous system that mature early, possibly the spinal cord.” [Emphases added.]
Student: Okay, what happened to fast-slow symmetry values?
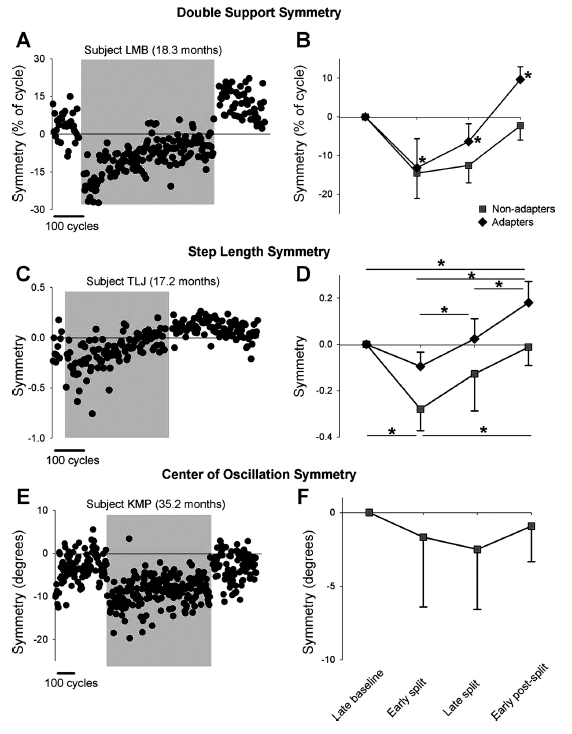
Professor: Below are the results for double support, step length, and center of oscillation symmetry as shown in Fig. 3 from Musselman et al. (2011):
Fig. 3. Symmetry of double support time, step length, and center of oscillation. A and C show data from single subjects who showed adaptation in double support and step length, respectively. E shows data from a single subject who did not show adaptation in center of oscillation. Data points represent means of 3 consecutive steps. Shaded region indicates the split-belt period. B, D, and F show group data for the adapters (solid diamonds) and nonadapters (solid squares) in measures of double support (n = 23 and 3, respectively), step length (n = 12 and 7, respectively), and center of oscillation (n=0 and 7, respectively). Values are means ± SD, after the asymmetry in baseline was removed (see METHODS). In B: *P < 0.008, significantly different from other time periods, including baseline. In D: *P <0.008, significant difference, with results for adapters and nonadapters shown at top and bottom of graph, respectively.
Professor: The investigators offered these comments,
“On exposure to split-belt walking, all but 1 child showed a change in double support time of the 2 legs (i.e., significant error), so 26 children were included in the analysis. After the early split period, the children showed one of two outcomes for double support throughout the remainder of the experiment. The first group, called adapters [23 / 26] showed a gradual return to symmetry by the end of the split-belt period. Moreover, a significant aftereffect was seen during the early postsplit period, with an asymmetry in the opposite direction from that seen in early split. … The second group, called nonadapters, did not show a return to symmetry by the end of the split-belt period and did not show an aftereffect [3 / 26]. Group data are shown in Fig. 3B.”
“Unlike double support time, several children (8/27) did not show any change in their step length with initial exposure to split-belt walking (i.e., no error). Of the remaining 19 children, adaptation of step length was seen in 12 children (adapter group), all of whom also showed adaptation with respect to time in double support…. In these children, the error in step length symmetry seen in the early split period gradually diminished over the course of the split-belt period. … Seven children did not show any adaptation in step length (i.e., no aftereffect, nonadapter group)…. In some of these children the error in step length symmetry seen in the early split period was reduced with further split-belt walking, whereas in others the size of the error remained unchanged throughout split-belt walking. Group data are shown in Fig. 3D.”
“Center of oscillation data was available for eight children, and most (7/8) showed a significant asymmetry (i.e., error) in the early split period. In two children, this error was reduced over the course of split-belt walking, but none of the children showed an aftereffect. Data from a single subject are shown in Fig. 3E, and group data are shown in Fig. 3F.”
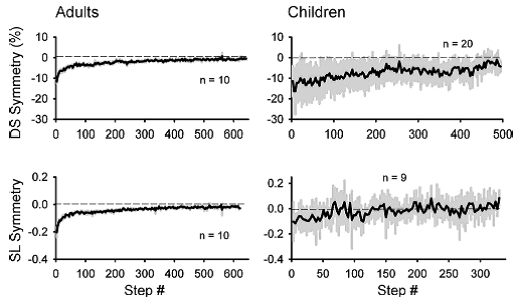
Professor: Next, Musselman et al. compared the time course of adaptation (i.e., learning) for children and adults as shown below in their Fig. 5:
Professor: The investigators also studied 10 adults using the same protocol. They noticed that,
“The time courses of adaptation for both double support and step length symmetry in adults and children are shown in Fig. 5. The adult data is best fit with a double-exponential function (r2 = 0.88 and 0.85 for double support and step length, respectively), whereas the data from the children is best fit with a straight line (r2 = 0.64 and 0.36 for double support and step length, respectively). Hence, the time course of adaptation in the early split period (i.e., first 40 steps) is slower in the children than in the adults.”
Professor: The authors began their discussion section indicating that,
- “We have shown that most children ages 8.5–36 mo of age adapt to split-belt walking. This adaptation was demonstrated
- as a reduction in the error in double support and step length symmetry caused by split-belt walking
- and the presence of an aftereffect on the return to tied belt walking. A few children showed adaptation in double support time, but not step length; none showed the reverse.
- No children showed adaptation in the spatial measure, center of oscillation.
- In addition, baseline asymmetry was seen more often in step length and center of oscillation compared with double support, suggesting that the neural mechanisms controlling spatial symmetry are different from those controlling temporal symmetry, in agreement with studies of patient populations (Choi et al. 2009).
- Furthermore, the time courses of adaptation to both double support and step length during early split-belt walking were slower in children compared with adults, suggesting that the neural mechanisms for adaptation are either different or not functioning in the same way. In contrast to the gradual emergence of adaptation in double support and step length, all children, regardless of age, showed immediate changes in stance time and stride length similar to those seen in adults (Reisman et al. 2005), indicating early maturation of the neural mechanisms controlling these measures.” [Emphases and formatting added.]
Professor: In their next subsection, entitled ” Neural mechanisms underlying split-belt walking and adaptation, Musselman et al. wrote:
“All children showed adultlike changes in measures of stride length and stance time during split-belt walking with no aftereffect during the early postsplit period (Reisman et al. 2005). Adults with diffuse cerebellar atrophy (Morton and Bastian 2006) and those with cerebral strokes (Reisman et al. 2007) also showed normal changes in these measures. … The duration of the stance phase and the excursion of the limb (i.e., stride length) are regulated by movement-related afferent feedback that affects phase transitions (i.e., stance to swing, swing to stance). For example, sensory inputs related to hip position and ankle loading regulate phase durations and transitions during locomotion (reviewed in Pearson 2008; Rossignol et al. 2006). Together, the results suggest that neural substrates controlling stance time and stride length in split-belt walking are contained in parts of the nervous system that mature early, possibly the spinal cord.
“Midline structures in the cerebellum may be especially important for split-belt adaptation, since cerebellar patients with gait and postural disturbances (functions controlled by midline cerebellar structures) were more impaired in split-belt adaptation than those with limb ataxia (functions controlled by intermediate and lateral cerebellar structures) (Morton and Bastian 2006). … Perhaps the midline cerebellar structures are not functionally mature in spite of the appearance of mature myelin. Alternatively, splitbelt adaptation may also depend on intermediate or lateral regions of the cerebellum, which are later to mature. For example, spatial adaptation may involve cerebral-cerebellar interactions, whereas temporal adaptation may involve spinal cord-cerebellar interactions (Malone and Bastian 2010; Vasudevan et al. 2011), which could explain the different ages at which spatial and temporal adaptation appear.
“Although the cerebellum is clearly important for split-belt adaptation, other neural structures cannot be discounted. For example, children with hemispherectomy show an impaired ability to adapt double support time but not step length, suggesting that the cerebrum may also play a role in temporal adaptation of split-belt walking (Choi et al. 2009). The basal ganglia are involved in many forms of motor learning, including motor adaptation (reviewed in Doyon et al. 2009), so its role in split-belt adaptation should be explored. The spinal cord may also have a role, since spinal animals are able to adapt their locomotor pattern in response to sustained force perturbations (Heng and de Leon 2007; Hodgson et al. 1994). Its role in split-belt adaptation remains to be determined.”
Professor: This early learning of coordinated adaptation to walking, each leg at a different speed, seems amazing. Bastian and her group has amply demonstrated that,
- “… the time courses of adaptation to both double support and step length during early split-belt walking [in this study] were slower in children compared with adults, suggesting that the neural mechanisms for adaptation are either different or not functioning in the same way.
- “In contrast to the gradual emergence of adaptation in double support and step length, all children, regardless of age, showed immediate changes in stance time and stride length similar to those seen in adults (Reisman et al. 2005), indicating early maturation of the neural mechanisms controlling these measures.”
- More importantly, Bastian and collegues have studied many patient groups, and their findings indicate that the ‘locomotor memory’ learning from split-belt walking is located in the spinal cord and brain stem, cerebellum, and possibly basal ganglia.
- They also reviewed their findings that impairments for children with hemispherectomy suggest that the cerebrum may also play a role in temporal adaptation of split-belt walking (Choi et al. 2009).
Student: Thus, my take home conclusion are that timing and placement aspects of locomotor memory is stored in the spinal cord, brainstem and cerebellum, with a likely involvement of the basal ganglia. Also, cerebral-cerebellar interactions may be involved. Hmm, isn’t the cortex where some of the motor memories are stored?
Professor: Excellent question! Okay. The next post shall look at that from the viewpoint of spinal cord: “Is only the spinal cord pattern-generation network reorganized during development?”
References
Choi JT, Vining EP, Reisman DS, Bastian AJ. Walking flexibility after hemispherectomy: split-belt treadmill adaptation and feedback control. Brain 132: 722–733, 2009.
Heng C, de Leon RD. The rodent lumbar spinal cord learns to correct errors in hindlimb coordination caused by viscous force perturbations during stepping. J Neurosci 27: 8558–8562, 2007.
Hodgson JA, Roy RR, de Leon R, Dobkin B, Edgerton VR. Can the mammalian lumbar spinal cord learn a motor task? Med Sci Sports Exerc 26: 1491–1497, 1994.
Malone LA, Bastian AJ. Thinking about walking: effects of conscious correction versus distraction on locomotor adaptation. J Neurophysiol 103: 1954–1962, 2010.
Morton SM, Bastian AJ. Cerebellar contributions to locomotor adaptation during split-belt treadmill walking. J Neurosci 26: 9107–9116, 2006.
Musselman KE, Patrick SK, Vasudevan EVL, Bastian AJ, Yang JF. Unique characteristics of motor adaptation during walking in young children. J Neurophysiol 105: 2195–2203, 2011.
Pearson KG. Role of sensory feedback in the control of stance duration in walking cats. Brain Res Rev 57: 222–227, 2008.
Reisman DS, Block HJ, Bastian AJ. Interlimb coordination during locomotion: what can be adapted and stored? J Neurophysiol 94: 2403–2415, 2005.
Reisman DS, Wityk R, Silver K, Bastian AJ. Locomotor adaptation on a split-belt treadmill can improve walking symmetry post-stroke. Brain 130: 1861–1872, 2007.
Rossignol S, Dubuc R, Gossard JP. Dynamic sensorimotor interactions in locomotion. Physiol Rev 86: 89–154, 2006.
Vasudevan EVL, Torres-Oviedo G, Morton SM, Yang JF, Bastian AJ. Younger is not always better: development of locomotor adaptation from childhood to adulthood. J Neurosci 31: 3055–3065, 2011.